Summary
- In November, Madrigal released open-label data from the MAESTRO-NAFLD study.
- This data was positive and could be used to make predictions about the entire study.
- Madrigal is a strong buy at current prices. Insiders should buy their own company's stock more.
- Looking for more investing ideas like this one? Get them exclusively at The Total Pharma Tracker. Get started today »
Madrigal Pharmaceuticals (MDGL) is a late-stage biopharma company developing resmetirom, a first-in-class, orally-administered, small-molecule, liver-directed, thyroid hormone receptor (THR)-? selective agonist. The drug targets a specific thyroid hormone receptor pathway in the liver, which is a "key regulatory mechanism common to a spectrum of cardio-metabolic and fatty liver diseases with a high unmet medical need." The asset is currently in two Phase 3 clinical studies, MAESTRO-NASH and MAESTRO-NAGLD-1, designed to demonstrate multiple benefits across a broad spectrum of NASH (non-alcoholic steatohepatitis) and NAFLD (non-alcoholic fatty liver disease) patients. I have covered Madrigal extensively before.
Madrigal's main catalyst is the set of two MAESTRO phase 3 trials, one each in NASH and NAFLD. The MAESTRO-NAFLD study, which is a "relatively lighter weight" study of "presumed NASH" patients in what has been termed by the company as a "real-life" NASH study with non-invasive monitoring of patient response, differs from the MAESTRO-NASH study in its selection of patients (less severe NASH, borderline NASH F1-F2 stages), study endpoints, and patient monitoring (biopsy vs non-biopsy). The two studies are best described in the following diagram:
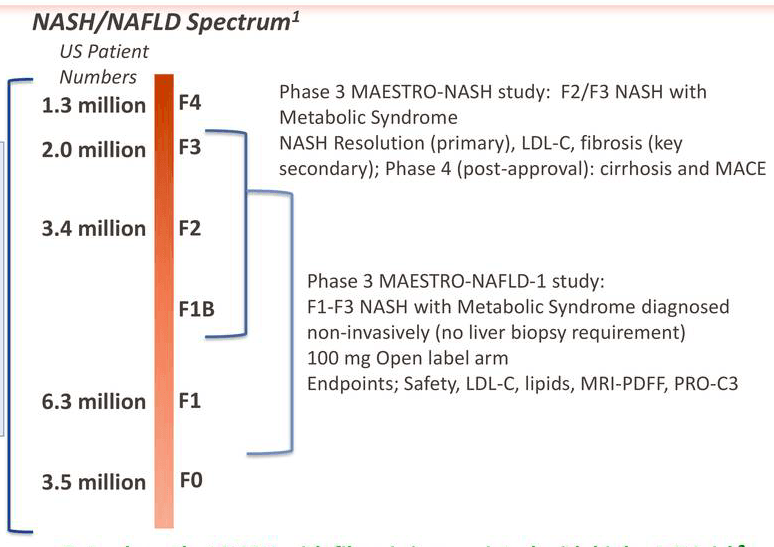
The NAFLD study will be completed by December 2021, while the NASH study by March 2024, with estimated primary completion date of December 2021. The NASH study began in March 2019, while the NAFLD study in December 2019. The NASH study, of course, targets more a severe disease form and therefore will be a crowning success for MGL-3196 or resmetirom, however the NAFLD study is no pushover either and targets a much higher earlier stage patient population.









