Summary
- INO is up over 300% year-to-date despite the recent sell off.
- INO-4800 got out of the blocks quickly, but the pace of development has faded a little.
- INO hasn't confirmed the details of its late-stage study of INO-4800, the market likely wants certainty that INO is still very much in this race.
When COVID-19 reared its head, of course vaccine-maker Inovio Pharmaceuticals (INO) threw its hat into the ring, like it does with each epidemic or pandemic. Is this time going to be different to the last? Or is INO stock set to continue fading.
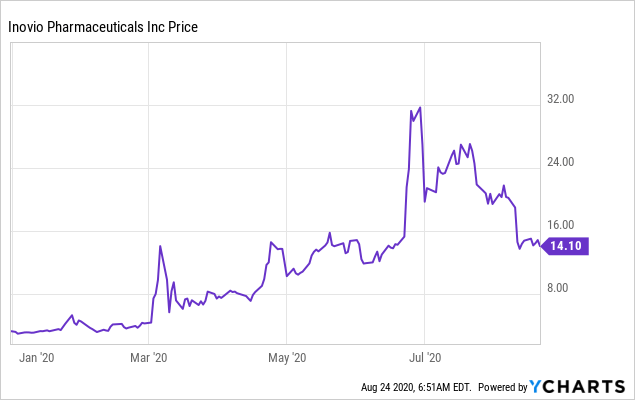 Data by YCharts
Data by YChartsFigure 1: Year-to-date trading of INO. Source: YCharts.
INO-4800
INO-4800 is a DNA vaccine, which comes with substantial advantages. The vaccine is very stable compared to some other vaccines, it doesn't require frozen storage or transport. DNA vaccines can be designed and produced quickly too. INO took full advantage of this and designed INO-4800 in early January, just a day or two after the genetic sequence for SARS-CoV-2 was available. By April INO started a 40 patient phase 1 study and in June/July began testing in older adults with another 80 participants added to that phase 1 study. A phase 1/2 study in South Korea was announced June 4, and results from the phase 1 study came at the end of June. Those results noted not only the development of binding and neutralizing antibodies but also T-cell responses. Seeking Alpha's Intrepid Investor has spoken recently about the importance of T-cell responses. Certainly INO-4800 is not to be dismissed, but there are some issues.
There are many competitors ahead
INO is planning to begin phase 2/3 study in September, but a number of competitors are ahead already. Those leading the charge in COVID-19 vaccine development are Moderna (MRNA), AstraZeneca (AZN) & Oxford University and Pfizer (PFE) & BioNTech (BNTX). Those three groups have made impressive progress on the rate of enrolment in studies with 10,000 patients. For example, MRNA announced on August 22 that they had enrolled 13,194 patients in their phase 3 study of mRNA-1273, the company's COVID-19 vaccine.
A look at the COVID-19 vaccine tracker can help us get an idea of where INO stands, how many other vaccines are ahead of INO's INO-4800. here are currently 11 vaccines in phase 3, phase 2/3 or phase 2 studies.









