Summary
Drugs that are shown to improve survival quickly become standard of care.
Tafamidis is the only drug known to improve survival in transthyretin-related (TTR) cardiac amyloidosis and is effective in both the hereditary type and much more common, acquired, "senile" type.
Tafamidis, a small molecule that is given by mouth, is considered less effective for hereditary amyloid polyneuropathy than the more complex, injectable patisiran and inotersen.
However, patisiran and inotersen, both orphan drugs, are not indicated for the more common, senile TTR amyloid.
Analysts already knew this. Perhaps they were not aware how large the addressable market for tafamidis could be?
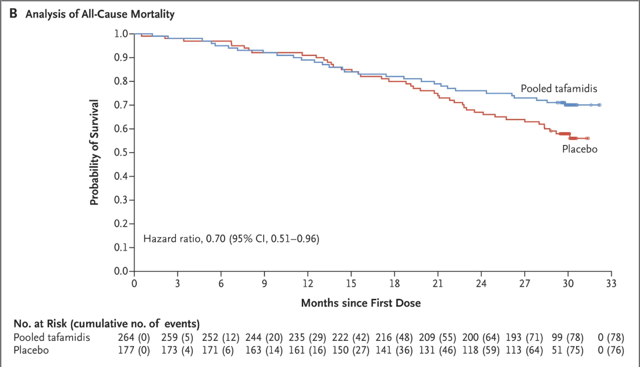
Novel drugs that are shown to improve survival in patients with a lethal condition quickly become standard of care for that condition. The ATTR-ACT Study sponsored by Pfizer (PFE) and published in Monday's issue of NEJMincludes survival curves for TTR (transthyretin-related) amyloid heart patients randomized to either tafamidis or placebo: